| In the IB Psychology Abnormal option we examine the effect of various biomedical approaches to the treatment of various psychological disorders. We examine the biomedical approach to the treatment of major depression under the following two learning outcomes:
There are different biomedical treatments for depression that could be considered here, for example lobotomies and electroshock (electroconvulsive) therapy, but by far the most widely used biomedical approach to the treatment of major depression is the use of antidepressants. You are probably familiar with the brand names Prozac and Zoloft even if you have never had a sad day in your life. The biological mechanisms behind our most common (and effective) antidepressants seem grounded in sound science. Serotonin is our brain's 'feel good' neurotransmitter, and by boosting serotonin levels in the brain we should be able to make our depressive patients feel a whole lot better about themselves and life in general. SSRIs, or selective serotonin re-uptake inhibitors, do exactly this, they inhibit the neurotransmitter serotonin from being reabsorbed back into the synapses where it was initially released, thus allowing for a build up of serotonin in the synaptic gap and an increase in activity in serotonergic neurons. What you will find once you dig deep enough is that this is incredibly controversial. | This question of biomedical treatments is examined in-depth in two model answers to the IB Psychology ERQs And, when we start prescribing these medications to our children in ever increasing numbers you can be sure that serious questions are going to be raised. Consider the list of side effects of one of our most commonly prescribed SSRIs: |
| The long, long side effects of Prozac list:
|
|
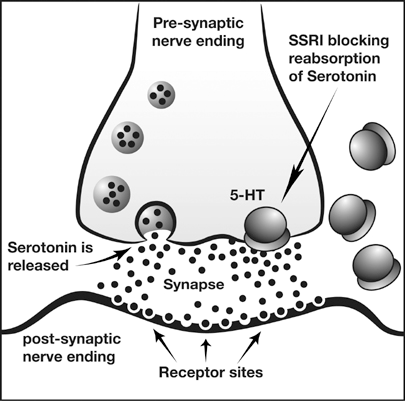
| Is the effectiveness of SSRIs due to the placebo effect? Reviewing the evidence we can conclude that using the most common antidepressant medications, the SSRIs such as Prozac and Zoloft are no more effective than taking a placebo. Moreover, reviewing the list of side effects, taking a placebo may be a whole lot safer! The biomedical treatment of depression The biomedical approach to treatment is based on the assumption that if a mental problem is caused by biological malfunctioning, the cure is to restore the biological system with drugs. For example, the serotonin hypothesis of depression suggests that depression is linked to low levels of the neurotransmitter serotonin (Coppen, 1967). Serotonin is a neurotransmitter produced by specific neurons in the brain that are called serotonergic neurons because they produce serotonin. Antidepressant treatment should therefore aim to regulate serotonin levels. Antidepressants are often used in the treatment of bulimia nervosa because many patients also suffer from other disorders such as depression (the problem of comorbidity). Antidepressants are also used to treat minor depressive symptoms but the American Food and Drug Administration (FDA, 2004) warned that the use of antidepressants for children and adolescents could perhaps lead to an increased risk of suicide. In fact, the FDA adopted a "black box" label warning indicating that antidepressants may increase the risk of suicidal thinking and behaviour in some children and adolescents with major depression at about twice the rate of placebo. A black-box warning is the most serious type of warning in prescription drug labelling. Selective serotonin reuptake inhibitors (SSRIs) Figure 1: SSRIs increase serotonin levels in the synaptic gap Antidepressants in the form of selective serotonin reuptake inhibitors (SSRI) block the reuptake process for serotonin This results in an increased amount of the serotonin in the synaptic gap (see figure 1). The theory is that this increases serotonergic nerve activity leading to an improvement in mood. Essentially, the only evidence that exists in favour of the serotonin hypothesis is the alleged efficacy of SSRIs – if they make your serotonin more potent and this improves your condition, the problem must have been in your serotonin levels to begin with, or so the logic goes. According to Lacoste & Leo (2005) this is an example of backward reasoning. Assumptions about the causes of depression are based on how people respond to a treatment and this is logically problematic. For example, the symptoms of headaches can be treated by aspirin, but this is definitely not to say that the cause of headaches is a deficiency of aspirin. SSRI drugs such as Prozac, Zoloft and Paxil are now amongst the most commonly prescribed antidepressants and this has been taken as indirect support for the serotonin hypothesis. They do affect mood and emotional responses positively in most people (although much of this may be due to the placebo effect; Kirsch et al., 2008). SSRIS have been criticised because they treat the symptoms of depression but do not cure the mental disorder, and because depressive episodes usually recur, it is necessary for patients to continue taking the medication. Unless the medication is used with therapy, it is unlikely that the disorder will disappear permanently. However, SSRIs are popular because they have fewer side effects than previous drugs such as tricyclic antidepressants. Not everyone can use SSRIs and the most common side effects are headache, nausea, sleeplessness, agitation and sexual problems. | Neale et al. (2011) conducted a meta-analysis of published studies on the outcome of antidepressants versus placebo. The study focussed on: (i) patients who started with antidepressants and then changed to placebo, (ii) patients who only received placebo, and (iii) patients who only took antidepressants. The study found that patients who do not take antidepressants have a 25% risk of relapse, compared to 42% or higher for those who have been on medication and then stopped it. According to the researchers, antidepressants may interfere with the brain’s self-regulation. They argue that drugs affecting serotonin or other neurotransmitters may increase the risk of relapse. The drugs reduce symptoms in the short-term but, when people stop taking the drug, depression may return because the brain’s natural self-regulation is disturbed. Ingeniously, Henninger et al. (1996) performed experiments where they reduced serotonin levels in healthy individuals to see if they would develop depressive symptoms. The results did not support that levels of serotonin could influence depression; i.e., there was no evidence for a cause-effect relationship, and they argued that it was necessary to revise the serotonin hypothesis. This is strong evidence against the hypothesis because if low levels of serotonin do cause depression and they were successful in reducing serotonin levels in their participants (and the evidence presented suggests that this was the case), then this can be considered strong evidence against the serotonin-depression hypothesis. However, there has been debate around just how depression was monitored in this study. Leauhter et al. (2002) examined changes in brain function during treatment with placebo. The study examined brain function in 51 patients with depression who either received placebo or an active antidepressant medication. An EEG was used to compare brain function in the two experimental groups. The design was double-blind and ran over none weeks. The study used two different SSRI, which were randomly allocated to participants. Results showed a significant increase in activity in the prefrontal cortex nearly from in the beginning in the trial in the placebo group. The pattern was different from the patients who were treated with the SSRI but patients in both groups got better. This indicates that medication is effective, but placebo is just as effective. The findings from the study are intriguing. The difference in activity in the brain indicates that the brain is perhaps able to heal itself since there was a positive effect for both groups. Believing that they are being treated could be enough for many patients. Kirsch et al. (2002) found that there was a publication bias in research into the effectiveness of SSRI in depression. In fact, if the results of all studies (including the ones that had not been published) were pooled together it would seem that the placebo effect accounted for 80% of the antidepressant response. A placebo is a substance that has no therapeutic effect, and is used as a control in testing new drugs. Of the studies funded by pharmaceutical companies, 57% failed to show a statistically significant difference between antidepressant and a neutral placebo. This and similar studies cast doubt on the serotonin hypothesis, not to mention the ethics of drug companies. However, it is still widely promoted by pharmaceutical companies and presumably believed by the 10% of Americans taking these SSRIs to treat depression. In sum, when evaluating the evidence for the biomedical approach to the treatment of major depression we can conclude that SSRIs may reduce depressive symptoms but they have side effects and do not cure patients. It is likely that the placebo effect could account for the effectiveness of the medication. Further, because the mechanisms are not well understood not how antidepressants affect the brain in the long-term, it is possible that the heavy use of these could well be damaging. There is also increasing criticism of the role of pharmaceutical companies and their marketing of antidepressants, which has led to an increase in the prescription of SSRI. So parents, your kid is acting a little bit moody, disinterested and disengaged from her surrounds, should you be pushing your GP for some Prozac? Have a read of the side effects of antidepressants in children and adolescents in the journal article below. |
Black Magic - The Placebo EffectAntidepressants are placebosBlack box warnings | side effects of SSRIs on childrenIf your answer to the above question was a resounding "No way!", based on the evidence that that they aren't effective, then you may have added reason to pat yourself on the back ... the FDA has also issued a 'black box' warning on all SSRI antidepressants. They found and believed it was in the public interest that those taking SSRIs be warned that there is an increased risk of suicidal thoughts or behavior (suicidality) in children and adolescents treated with SSRI antidepressant medications. The black box warning, a type of warning issued by the FDA that must appear on the packaging of certain prescription drugs. The U.S. Food and Drug Administration (FDA) has required all pharmaceutical companies to place a boxed warning on the labeling of prescription antidepressants, or in literature describing it. It is the strongest warning that the FDA requires, and signifies that medical studies indicate that the drug carries a significant risk of serious or even life-threatening adverse effects. In this instance, the FDA believes that already depressed children are more likely to commit suicide because taking these medications leads to the idea of suicide becoming more salient. Consequently, prescriptions issued for antidepresants have fallen by over 20 per cent in the US. Now, this may seem a good thing in light of all of the evidence presented here. But consider this: during this time, child and adolescent suicides in the US jumped by 11 per cent! So, I'll leave you with this final thought ... should we now be prescribing a nice, safe placebo instead for a mental illness that has very real consequences for the sufferer? |





 RSS Feed
RSS Feed